Lyme Disease Clinical Trials
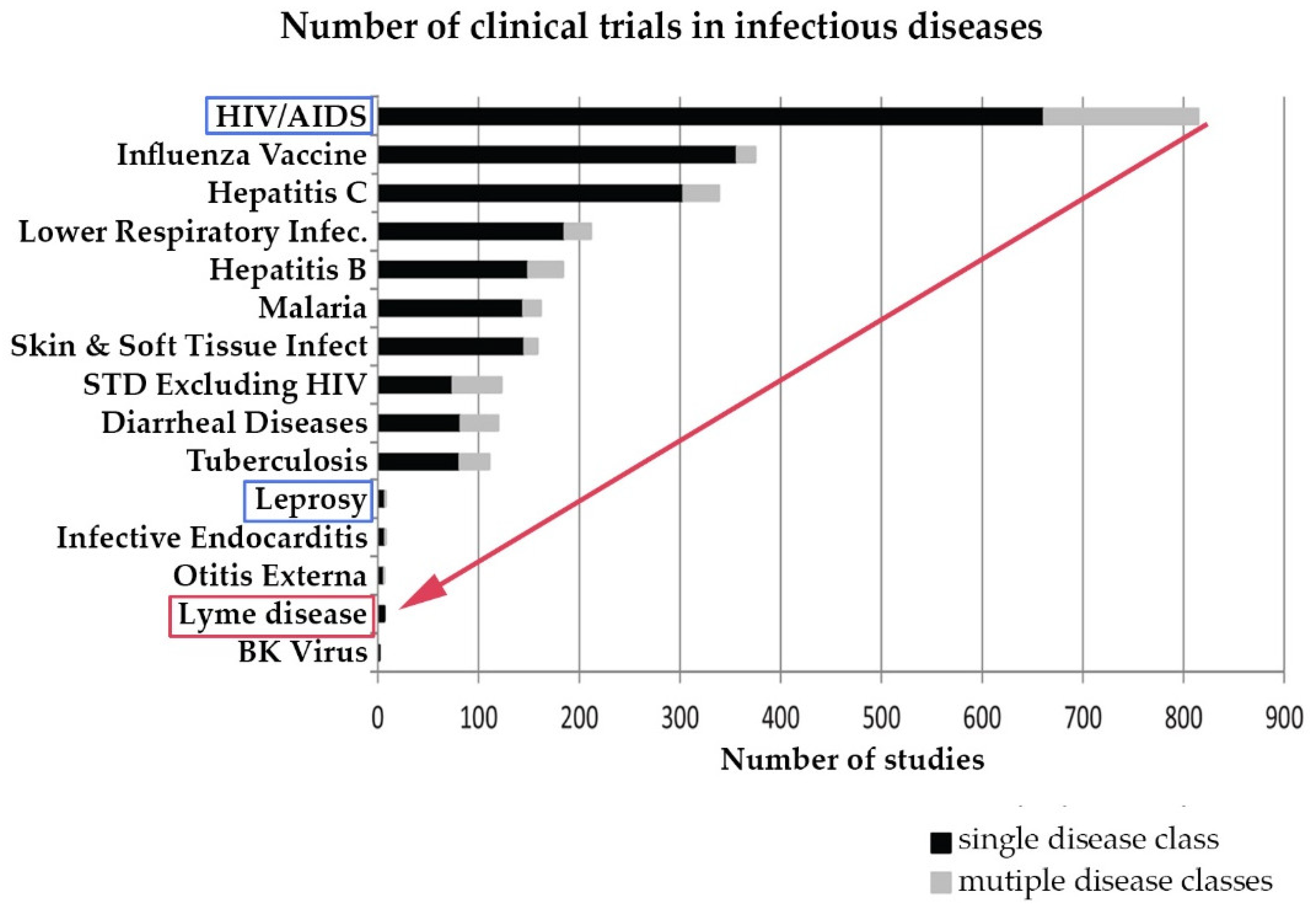
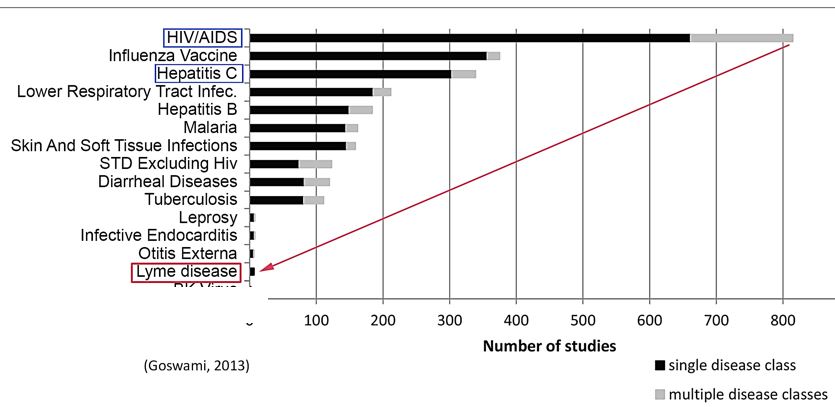
Lyme disease clinical trials. Experts are warning of an uptick in ticks this year - and that could increase the chances of Lyme Disease being spread. There are 22 standardized instruments used to measure the severity of PLDS among the four published National Institutes of Health NIH sponsored double-blind randomized placebo-controlled trials RCTs. Clinical trials validate the severity of persistent Lyme disease symptoms.
A biostatistical review of randomized placebo-controlled clinical trials Contemp Clin Trials. Lyme disease is a disease carried by infected ticks and can cause the infection to spread to the joints heart and nervous system 0 views. Rajadas and Pothineni have patented the compound for the treatment of Lyme disease and are working with a company to develop an oral form of the drug.
NIAID has funded three placebo-controlled clinical trials to learn more about the efficacy of prolonged antibiotic therapy for treating PLDS. To examine the impact of Lyme disease on patients immune systems and their long-term health. EVALUATION OF THE HYPOTHESIS.
As director of the new clinical research center housed at Johns Hopkins Bayview Medical Center Aucott has received an initial grant to lead the first prospective controlled study in the US. Within the study 600 healthy subjects aged 5-65 years will be included. Study duration per subject will be a maximum of 19 months in Part A and additional 37 months for subjects enrolled in the Part B.
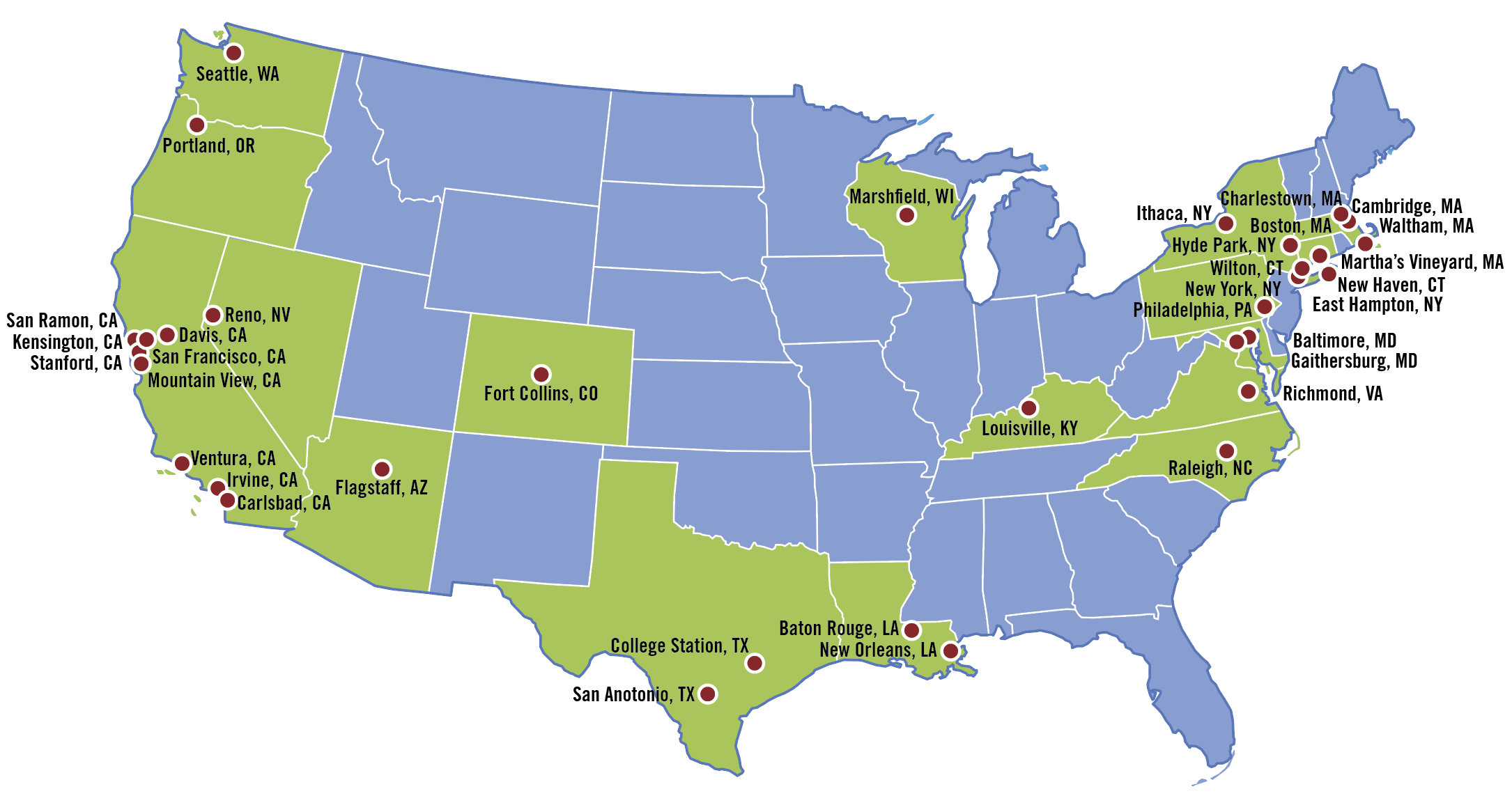
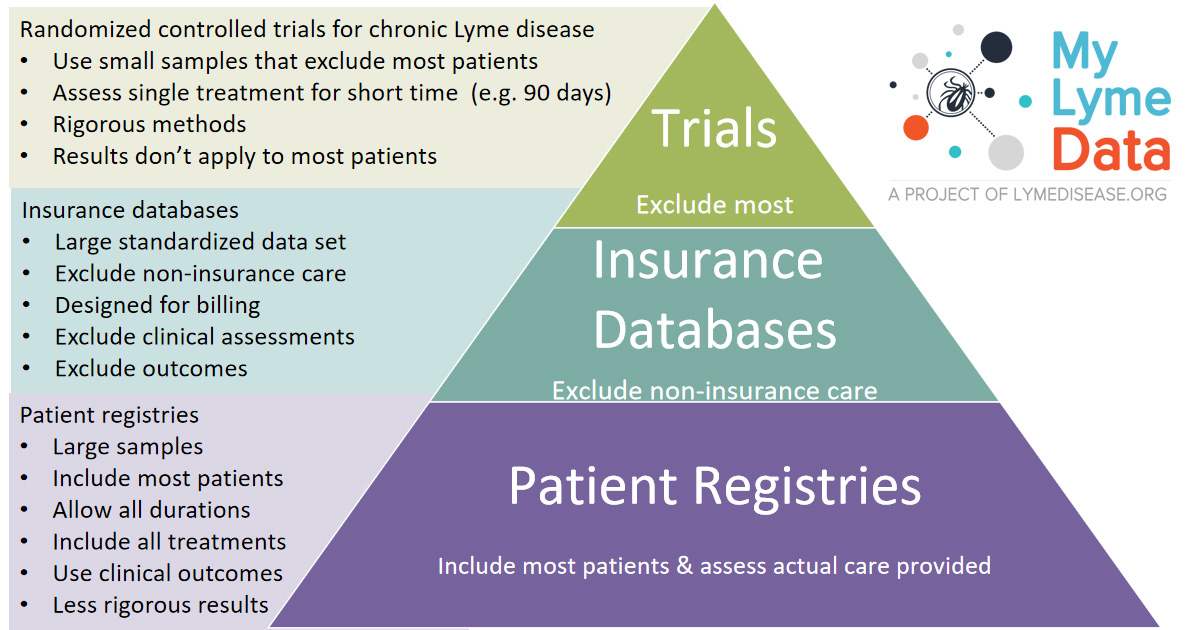
The published results were. Clinical trials network center seeks input from Lyme patients by July 1 In recognition of a severe unmet need the Steven and Alexandra Cohen Foundations grant to Columbia University established the first national Clinical Trials Network Coordinating Center CTNCC for Lyme and other Tick-borne Diseases. The following are selected clinical trials sponsored by NIAID and researching various aspects of Lyme disease.
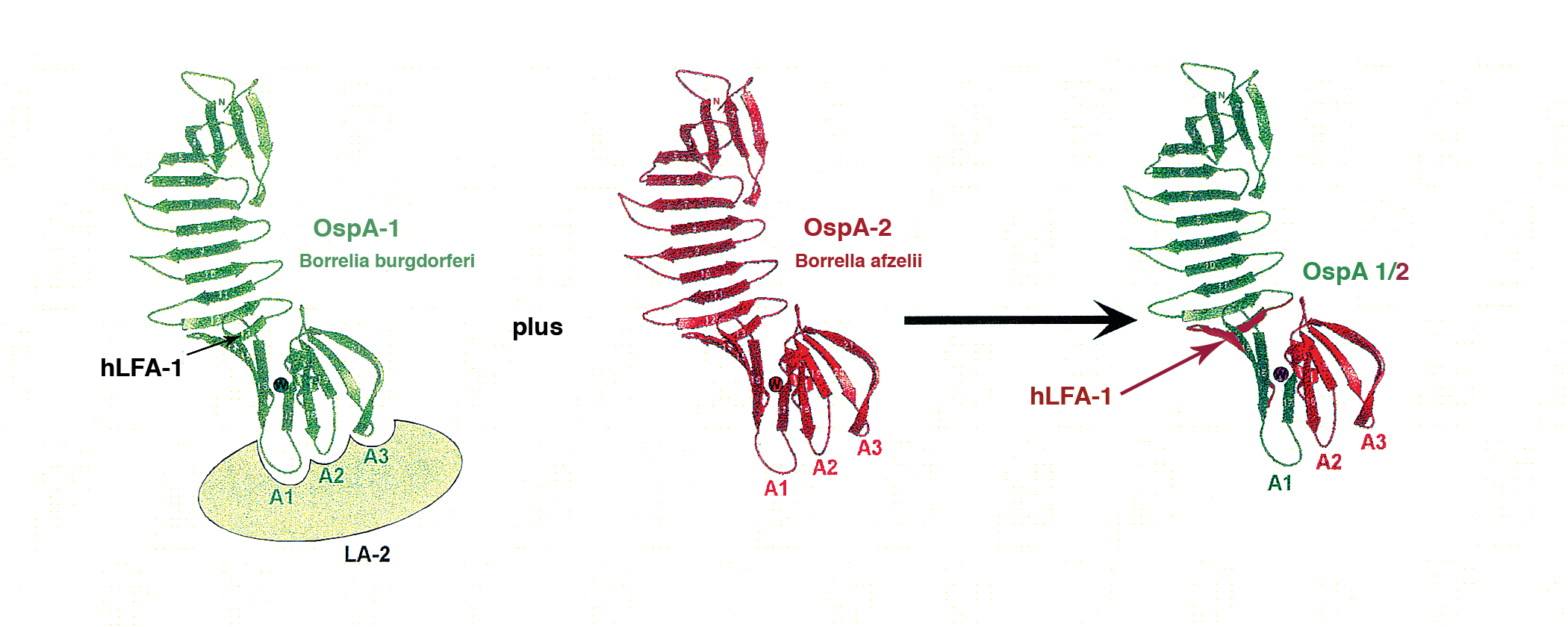
First Clinical Study of the Safety and Blood Levels of a Human Monoclonal Antibody 2217LS Against Lyme Disease Bacteria in Healthy People is human antibody designed to provide protection from Lyme disease. Lyme disease vaccine in clinical trials good news for everyone Even though dogs have access to a Lyme disease vaccine there has not been a vaccine available for humans for 18 years. Researchers plan to conduct a clinical trial.
For more information about the studies you may click on the links call 1-800-411-1222 or email lymedxstudiesniaidnihgov. Although azlocillin is an FDA-approved drug more research needs to be done before it is used to treat Lyme patients.
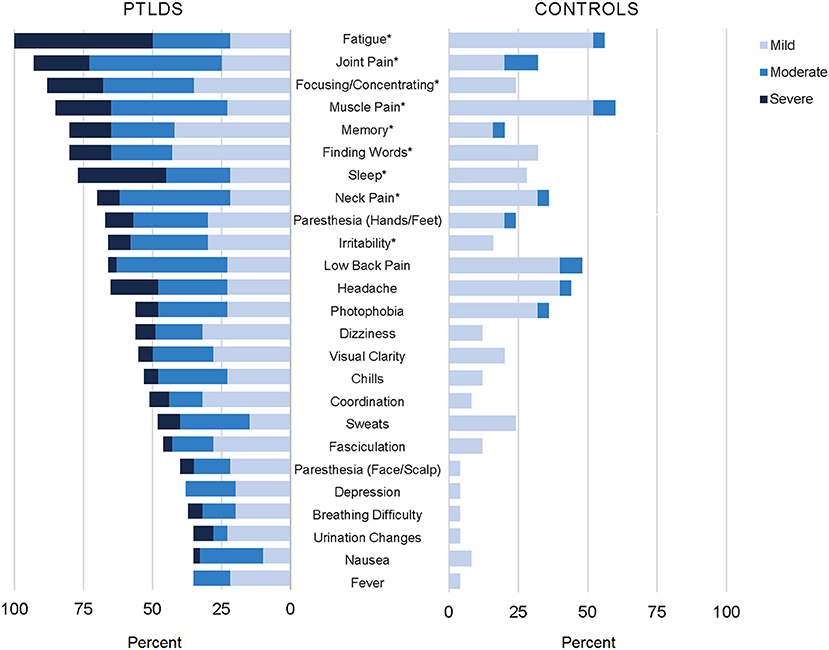
Clinical trials validate the severity of persistent Lyme disease symptoms.
The authors of 4 National Institutes of Health-sponsored antibiotic treatment trials of patients with persistent unexplained symptoms despite previous antibiotic treatment of Lyme disease determined that retreatment provides little if any benefit and carries significant risk. Within the study 600 healthy subjects aged 5-65 years will be included. The following are selected clinical trials sponsored by NIAID and researching various aspects of Lyme disease. Antibiotic retreatment of Lyme disease in patients with persistent symptoms. The published results were. First Clinical Study of the Safety and Blood Levels of a Human Monoclonal Antibody 2217LS Against Lyme Disease Bacteria in Healthy People is human antibody designed to provide protection from Lyme disease. As director of the new clinical research center housed at Johns Hopkins Bayview Medical Center Aucott has received an initial grant to lead the first prospective controlled study in the US. There are 22 standardized instruments used to measure the severity of PLDS among the four published National Institutes of Health NIH sponsored double-blind randomized placebo-controlled trials RCTs. EVALUATION OF THE HYPOTHESIS.
To examine the impact of Lyme disease on patients immune systems and their long-term health. Study duration per subject will be a maximum of 19 months in Part A and additional 37 months for subjects enrolled in the Part B. Although azlocillin is an FDA-approved drug more research needs to be done before it is used to treat Lyme patients. To examine the impact of Lyme disease on patients immune systems and their long-term health. For the purposes of this study a recovered control is defined as an otherwise healthy male or female aged 18 and above who has had Lyme disease fulfilling the CDC Lyme Disease National Surveillance Case Definition and who had received accepted antibiotic treatment for Lyme disease at least 3 months since the end of antibiotic therapy before protocol evaluation and. There are 22 standardized instruments used to measure the severity of PLDS among the four published National Institutes of Health NIH sponsored double-blind randomized placebo-controlled trials RCTs. Evaluation Treatment and Follow-up.





























-958222134_MCRI.jpg)






Post a Comment for "Lyme Disease Clinical Trials"